More Post from the Author
- Chelsea Football Club selects IFS as Principal Partner
- Zillow and Google bring home-buying guidance to NotebookLM
- Redwood Software redfinit l'observabilit de l'automatisation avec Redwood Insights Premium
- Liftoff Announces Confidential Submission of Draft Registration Statement for Proposed Initial Public Offering
- /C O R R E C T I O N -- Educate 360/
Biologics CDMO Market to Grow by USD 16.32 Billion (2025-2029), Driven by Cost-Efficient Resources in Emerging Markets, AI Impact on Trends - Technavio
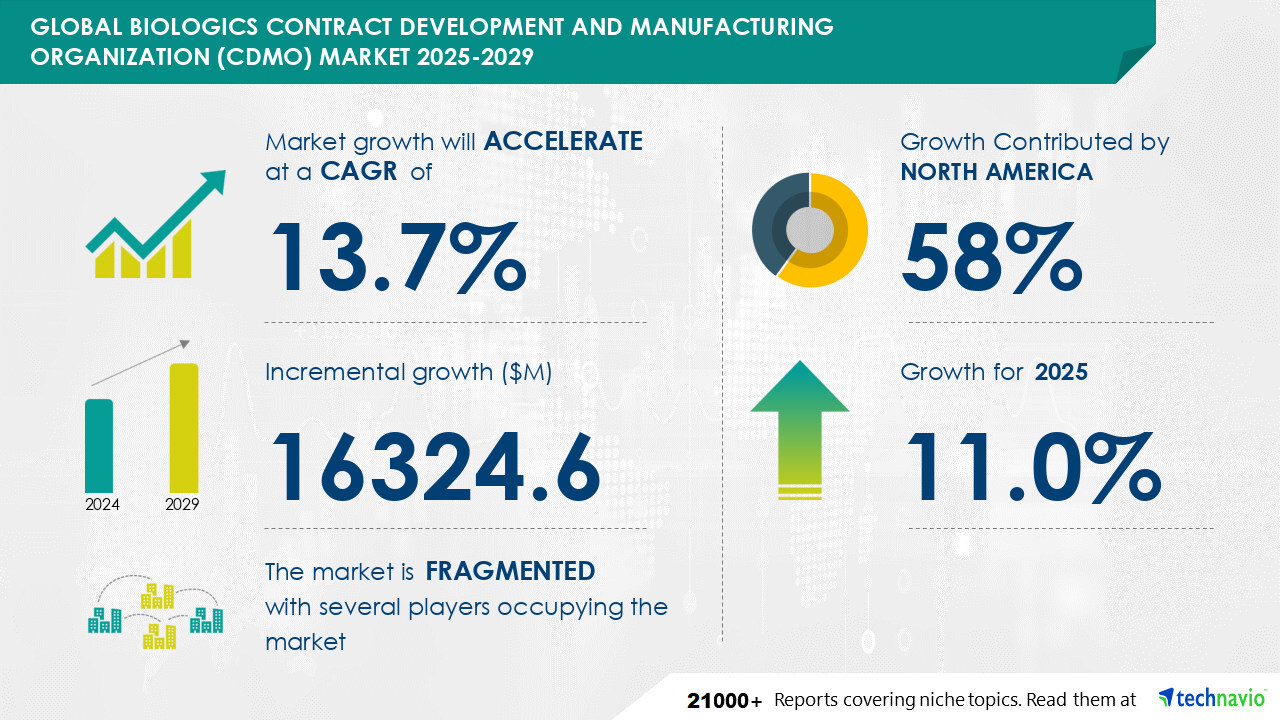
NEW YORK, Feb. 4, 2025 /PRNewswire/ -- Report on how AI is redefining market landscape - The globalbiologics contract development and manufacturing organization (CDMO) marketsize is estimated to grow by USD 16.32 billion from 2025-2029, according to Technavio. The market is estimated to grow at a CAGR of 13.7% during the forecast period. Availability of cost-efficient resources in emerging marketsis driving market growth,with a trend towardsadvent of big data. However,capacity utilization and constraints poses a challenge. Key market players include 3P Biopharmaceuticals, AbbVie Inc., AGC Biologics, Binex Co. Ltd., Boehringer Ingelheim International GmbH, Bora Pharmaceuticals, Catalent Inc., Evonik Industries AG, FUJIFILM Corp., Grifols SA, J RETTENMAIER and SOHNE GmbH and Co KG, JSR Corp., Kemwell Biopharma Pvt. Ltd., Lonza Group Ltd., Novartis AG, Rentschler Biopharma SE, Samsung Electronics Co. Ltd., Shenzhen Hepalink Pharmaceutical Co. Ltd., Toyobo Co. Ltd., and WuXi Biologics Cayman Inc..
Key insights into market evolution with AI-powered analysis. Explore trends, segmentation, and growth drivers- View Free Sample PDF
Biologics Contract Development And Manufacturing Organization (CDMO) Market Scope | |
Report Coverage | Details |
Base year | 2024 |
Historic period | 2019 - 2023 |
Forecast period | 2025-2029 |
Growth momentum & CAGR | Accelerate at a CAGR of 13.7% |
Market growth 2025-2029 | USD 16324.6 million |
Market structure | Fragmented |
YoY growth 2022-2023 (%) | 11.0 |
Regional analysis | North America, Europe, Asia, and Rest of World (ROW) |
Performing market contribution | North America at 58% |
Key countries | US, Germany, Canada, France, China, UK, Japan, Italy, India, and South Korea |
Key companies profiled | 3P Biopharmaceuticals, AbbVie Inc., AGC Biologics, Binex Co. Ltd., Boehringer Ingelheim International GmbH, Bora Pharmaceuticals, Catalent Inc., Evonik Industries AG, FUJIFILM Corp., Grifols SA, J RETTENMAIER and SOHNE GmbH and Co KG, JSR Corp., Kemwell Biopharma Pvt. Ltd., Lonza Group Ltd., Novartis AG, Rentschler Biopharma SE, Samsung Electronics Co. Ltd., Shenzhen Hepalink Pharmaceutical Co. Ltd., Toyobo Co. Ltd., and WuXi Biologics Cayman Inc. |
Market Driver
The Biologics Contract Development and Manufacturing Organization (CDMO) market is experiencing significant growth, particularly in the biologics segment, which includes monoclonal antibodies, immunotherapies, and vaccines. Small molecules continue to dominate the pharma industry, but the trend is shifting towards large molecules like biologics, biosimilars, and cancer therapies. CDMOs specialize in various niches, such as biopharmaceutical CDMOs for recombinant proteins and vaccines, and niche CDMOs for advanced technology like plant-based expression systems and cell receptors. The supply chain is crucial, with quality testing, third-party logistic providers, and automation playing key roles. Advanced technology and specialized expertise are essential for producing complex biological medications, such as monoclonal antibodies and immunotherapies. The disease landscape is vast, from infectious diseases and chronic conditions like diabetes to geriatric population health issues. CDMOs offer clinical services, process engineering, and cell bank creation to help pharma clients navigate the development and manufacturing of biological medications. The biologics CDMO market is driven by the need for cutting-edge technology, evidence-based practice, and the production of novel therapies for various indications, including cancer, infectious diseases, and metabolic illnesses. CDMOs provide expertise in fermentation, microbial fermentation, tissue processing, and cell line engineering. Challenges like myelosuppression, cardiotoxicity, renal insufficiency, and neurotoxicity require careful consideration during the development and manufacturing process. Additionally, the aging population and polypharmacy present unique challenges in managing homeostatic processes and metabolism, particularly in the context of hepatic metabolism and renal excretion. CDMOs offer solutions for these challenges using mammalian cell lines, proteins, and biological medications. In conclusion, the biologics CDMO market is a dynamic and complex industry, requiring specialized expertise, advanced technology, and a deep understanding of various disease areas and therapeutic indications. CDMOs play a crucial role in the development and manufacturing of biological medications, from small-molecule drugs to large-molecule biologics, vaccinations, and gene therapy. The market is driven by the need for innovative solutions to address the challenges of producing complex biological medications while ensuring quality, safety, and efficacy.
The pharmaceutical industry's global advancements in drug development technology extend beyond research and development. Analytical tools are increasingly utilized for clinical data synthesis, expediting the drug development process. Big data is a popular strategy in healthcare, including pharmaceuticals, to aid in research and development decision-making. Data analytics effectively identifies new drug candidates, predicts drug efficacy through simulation models, pinpoints patient populations, and monitors potential side effects for early avoidance. Biologics Contract Development and Manufacturing Organizations (CDMOs) leverage these tools to streamline their services, ensuring efficient and effective drug development for their clients.
Request Sampleof our comprehensive report now to stay ahead in the AI-driven market evolution!
- The Biologics Contract Development and Manufacturing Organization (CDMO) market encompasses small molecules, large molecules, and biologics, including biosimilars, cancer therapies, monoclonal antibodies, and immunotherapies. Biologic CDMOs specialize in advanced technology and specialized expertise, catering to the needs of biopharmaceutical companies. Challenges in this sector include supply chain complexities, quality testing, and collaboration with third-party logistic providers. The Biologics segment, consisting of monoclonal antibodies, immunotherapy, and recombinant proteins, presents unique challenges. For instance, the production of monoclonal antibodies requires fermentation, microbial fermentation, and cell line engineering. Niche CDMOs focus on infectious diseases, while biopharmaceutical CDMOs tackle chronic diseases like diabetes and ageing, which impact homeostatic processes in the geriatric population. Biologics CDMOs offer clinical services, automation, software development, and process engineering. They help pharmaceutical clients navigate challenges such as myelosuppression, cardiotoxicity, renal insufficiency, neurotoxicity, polypharmacy, and mental state. Advanced technology and evidence-based practice are crucial in addressing these challenges. The production of biologics involves mammalian cell lines, proteins, and biological medications. Vaccinations, somatic cells, tissues, and gene therapy also fall under this category. Allergies and metabolic illnesses, including metabolic syndrome, require specialized attention. Biologics CDMOs employ cutting-edge technology and specialized expertise to overcome these challenges and bring novel therapies to market.
- The biologics Contract Development and Manufacturing Organization (CDMO) market is currently challenged by capacity utilization issues. This measure evaluates actual production against a company's maximum production capacity. Capacities are crucial for producing various therapeutics, particularly biological drugs, due to their intricate manufacturing processes. Approximately 35% of CDMOs encounter minor capacity utilization constraints, while 20% face moderate to significant challenges during manufacturing. These constraints hinder CDMOs from reaching their full production potential, causing delays in the release of numerous therapeutics.
Discover how AI is revolutionizing market trends-Get your access now!
This biologics contract development and manufacturing organization (cdmo) market report extensively covers market segmentation by
- Type
- Mammalian
- Microbial
- Product Type
- Biologics
- Biosimilars
- Geography
- North America
- Europe
- Asia
- Rest Of World (ROW)
- Service
- CMO
- CRO
1.1Mammalian- The global biologics contract development and manufacturing organization (CDMO) market is significantly driven by the use of mammalian cells in the production of therapeutic proteins. Mammalian cells, derived from mammalian tissue, are primarily used for growing animal cells in vitro, in flasks or dishes. These cells include fibroblasts, epithelial cells, lymphocytes, and macrophages. Lymphocytes are found in the blood, while the rest are found in tissue. The major share of the market in 2024 is attributed to this segment due to the increasing application of mammalian cells in treating various diseases. Mammalian cells are used for producing human proteins with high therapeutic potential, such as tissue plasminogen activators, clotting factors, and erythropoietin. These proteins are produced through mammalian cell culture, which is also used for producing vaccines in bulk. The use of mammalian cell culture technology in vaccine production is a significant revenue generator in this market. Catalent Inc., a leading CDMO, recently signed an agreement with Spicona to develop a virus-like protein-based vaccine against COVID-19 using its proprietary GPEx cell line development technology. Catalent provides comprehensive services ranging from preclinical to commercial stages, including mammalian cell line development, process development, process validation, formulation development, and drug substance manufacturing. These factors are expected to drive the growth of the mammalian segment in the global biologics CDMO market during the forecast period.
Download a Sampleof our comprehensive report today to discover how AI-driven innovations are reshaping competitive dynamics
The Biologics Contract Development and Manufacturing Organization (CDMO) market encompasses the production and development of large molecule therapeutics, including biologics, monoclonal antibodies, biosimilars, and cancer therapies, for pharma clients. This market caters to various sectors, such as chronic infectious diseases and diabetes patients, and offers services from small-molecule drugs to clinical trials and commercial manufacturing. Biologics CDMOs provide supply chain solutions, quality testing, and regulatory compliance, ensuring the production of high-quality therapeutics. These organizations leverage advanced technologies like automation, software development, and process engineering to streamline production and improve efficiency. Moreover, they offer clinical services, tissue processing, and expertise in biology and biopharmaceuticals to meet the unique needs of their clients.
The Biologics Contract Development and Manufacturing Organization (CDMO) market encompasses the production of various types of biologics, including small molecules, large molecules, biosimilars, monoclonal antibodies, cancer therapies, and vaccines. Advanced technology and specialized expertise are crucial in this sector, with a focus on biopharmaceuticals, fermentation, microbial fermentation, and plant-based expression systems. The Biologics segment includes recombinant proteins, monoclonal antibodies, and immunotherapy, while the Monoclonal segment covers antibodies and cell receptors. The market caters to various disease landscapes, including chronic infectious diseases, cancer, and geriatric population-related conditions such as myelosuppression, cardiotoxicity, renal insufficiency, neurotoxicity, polypharmacy, and mental state disorders. Biologics CDMOs provide clinical services, automation, software development, process engineering, and biology expertise, enabling the production of biological medications, vaccinations, somatic cells, tissues, and novel therapies based on nucleic acids and gene therapy. The market also addresses allergies and metabolic illnesses, such as diabetes and metabolic syndrome. The supply chain involves third-party logistic providers to ensure the timely delivery of high-quality products. Evidence-based practice and quality testing are essential in maintaining the integrity and efficacy of these complex biological molecules.
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- Type
- Mammalian
- Microbial
- Product Type
- Biologics
- Biosimilars
- Geography
- North America
- Europe
- Asia
- Rest Of World (ROW)
- Service
- CMO
- CRO
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email:[emailprotected]
Website:www.technavio.com/
SOURCE Technavio

More Post from the Author
- Chelsea Football Club selects IFS as Principal Partner
- Zillow and Google bring home-buying guidance to NotebookLM
- Redwood Software redfinit l'observabilit de l'automatisation avec Redwood Insights Premium
- Liftoff Announces Confidential Submission of Draft Registration Statement for Proposed Initial Public Offering
- /C O R R E C T I O N -- Educate 360/

